| Home |
| Green Building Bible, Fourth Edition |

|
These two books are the perfect starting place to help you get to grips with one of the most vitally important aspects of our society - our homes and living environment. PLEASE NOTE: A download link for Volume 1 will be sent to you by email and Volume 2 will be sent to you by post as a book. |
Vanilla 1.0.3 is a product of Lussumo. More Information: Documentation, Community Support.
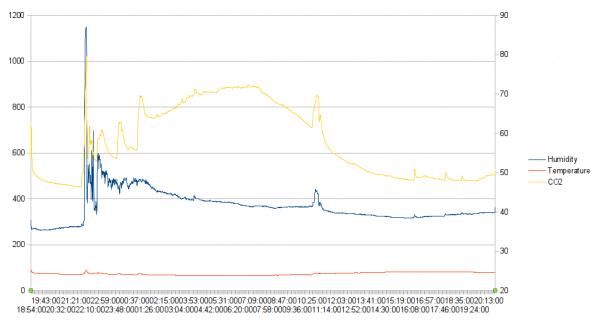
Posted By: Simon StillWould 1 person in a small room be enough to spike CO2 that much?

 Or my arithmetic has gone astray.
Or my arithmetic has gone astray.
Posted By: SteamyTeaJust randomly thinking here, but could the CO2 be coming out the hot water. Warmer water can hold more CO2
I may be talking out my rear end, or that may be the problem.
Our old mate Damon HD did some research into this, I think the data is on his website.
Posted By: Ed DaviesAre you sure? Normally gases come out of solution as water warms.Your dead right, I realised as I was driving to work I had it the wrong way around.
Posted By: willie.macleodCO2 sensors will spike with someone standing next to them and show similar readings to what you are getting.
Your wife probably completely ignored the thing and you probably looked over at it because you were aware it was there and breathed in its direction a few times.....
Sensor placement is crucial and avoiding putting them in direct line of folk breathing next to them. In a very small room you would need to be even more careful.
Posted By: Ed DaviesAre you sure? Normally gases come out of solution as water warmsBut wouldn't warm water be a better solvent, and if prevented from off-gassing (enclosed in pipe) could be carrying more CO2 until released to fresh air?
1 to 12 of 12